Two reviews from our group summarise the latest efforts in organ-on-chip technologies to emulate in vitro microfluidic systems. These devices are an opportunity to evolve the fields of biofabrication and sensing technology
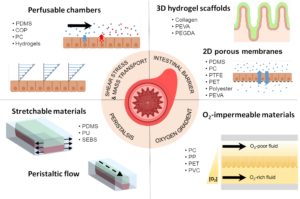
Organ-on-chip (OOC) technology has been an efficient tool in modern research to substitute laboratory mice and simulate tissue and organ-level physiology and function. In particular, these in vitro devices have been extensively applied to model the intestine, enhancing the research community’s knowledge about intestinal physiology and pathophysiology in order to develop targeted therapies for a more precise and personalised treatment of intestinal diseases.
Now, a review published in Biosensors & Bioelectronics signed by GAB members, collects information about the intestine models and highlights the necessity to integrate sensors into these in vitro models to shine light on the pathological mechanisms of intestinal disorders at their early stage. The detection of a disease at its early state would allow more efficient treatments and a better prognosis, reducing costs and enhancing the quality of life of the patients.
Last years’ research has had a significant impact in these complex microfluidic systems, though there is still a long way to go to increase biosensors capacity in their operations.
«The potential of the OOC technology is enormous. OOC technology may provide a true precision medicine, allowing the use of the patients’ own cells for performing drugs screening before treating the patient», explains Mar Álvarez, GAB member and one of the authors. «To that end, we believe that the integration of sensors into this platforms is mandatory to understand and evaluate the functioning of the organ in real time, providing information that may be used for in-situ decision making», she continues.
Hydrogel microfluidic platforms to improve the predictive capacities of the in vitro models
Another GAB review article published in Applied Materials & Interfaces tackles the progress made in tissue barrier models, as they have a crucial role in regulating organ homeostasis. Current microfluidic systems do not properly mimic cells’ interaction, so recent developments have included biomaterials, such as hydrogels, to emulate these boundaries between tissues and external environment. A hydrogel acts as a microenvironment of the cell and it permits cell culture.
«The hydrogel mimics the real cell microenvironment, providing the mechanical cues needed to reproduce the proper organ physiology and function», Álvarez adds.
Recent developments in the fields of biofabrication show that hydrogels are able to mimic and change the tissue properties and dynamics, thus enabling an in vivo recreation for its reparation.
09/04/2021