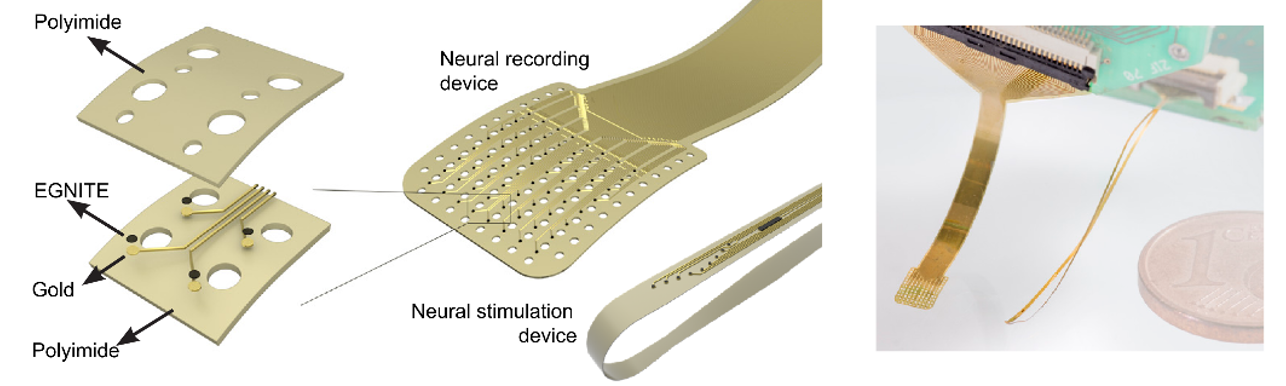
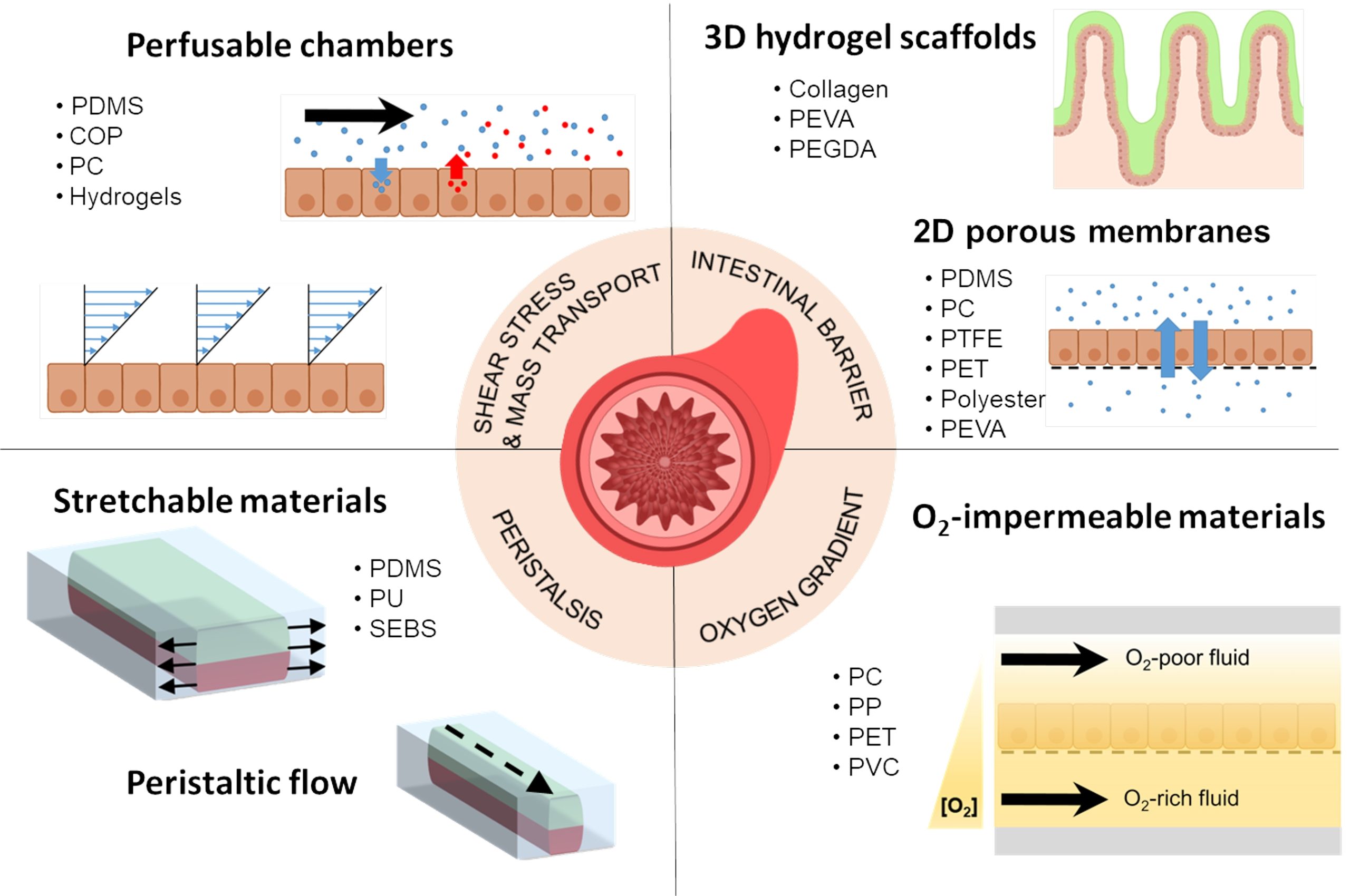
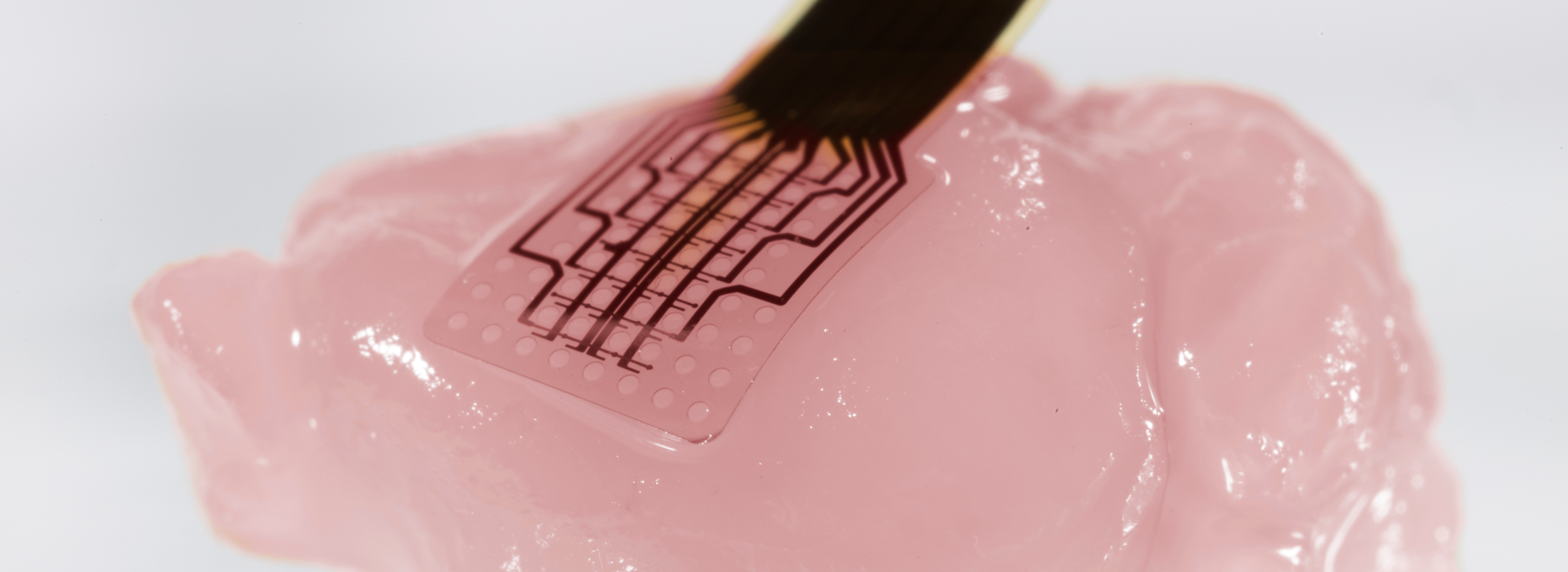
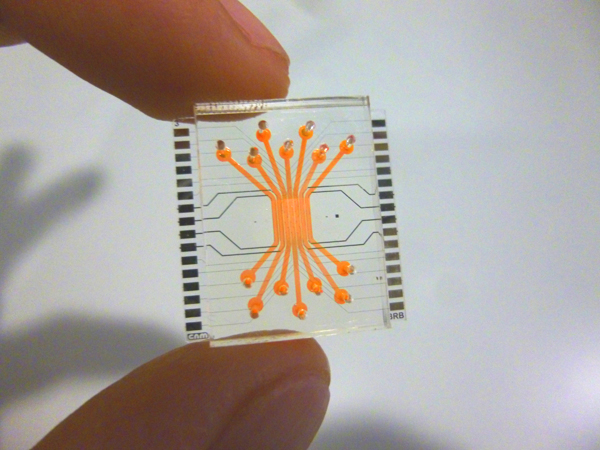
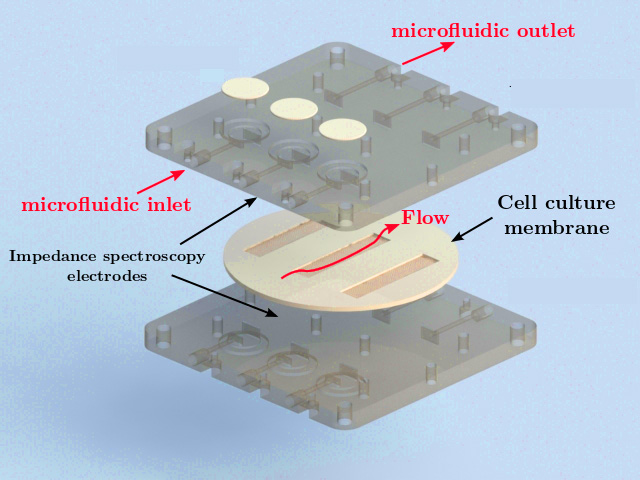
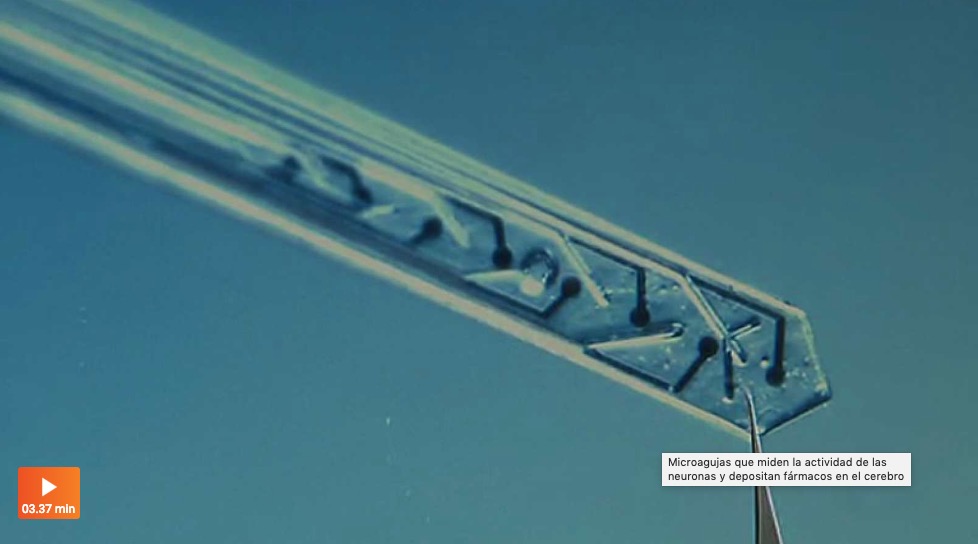
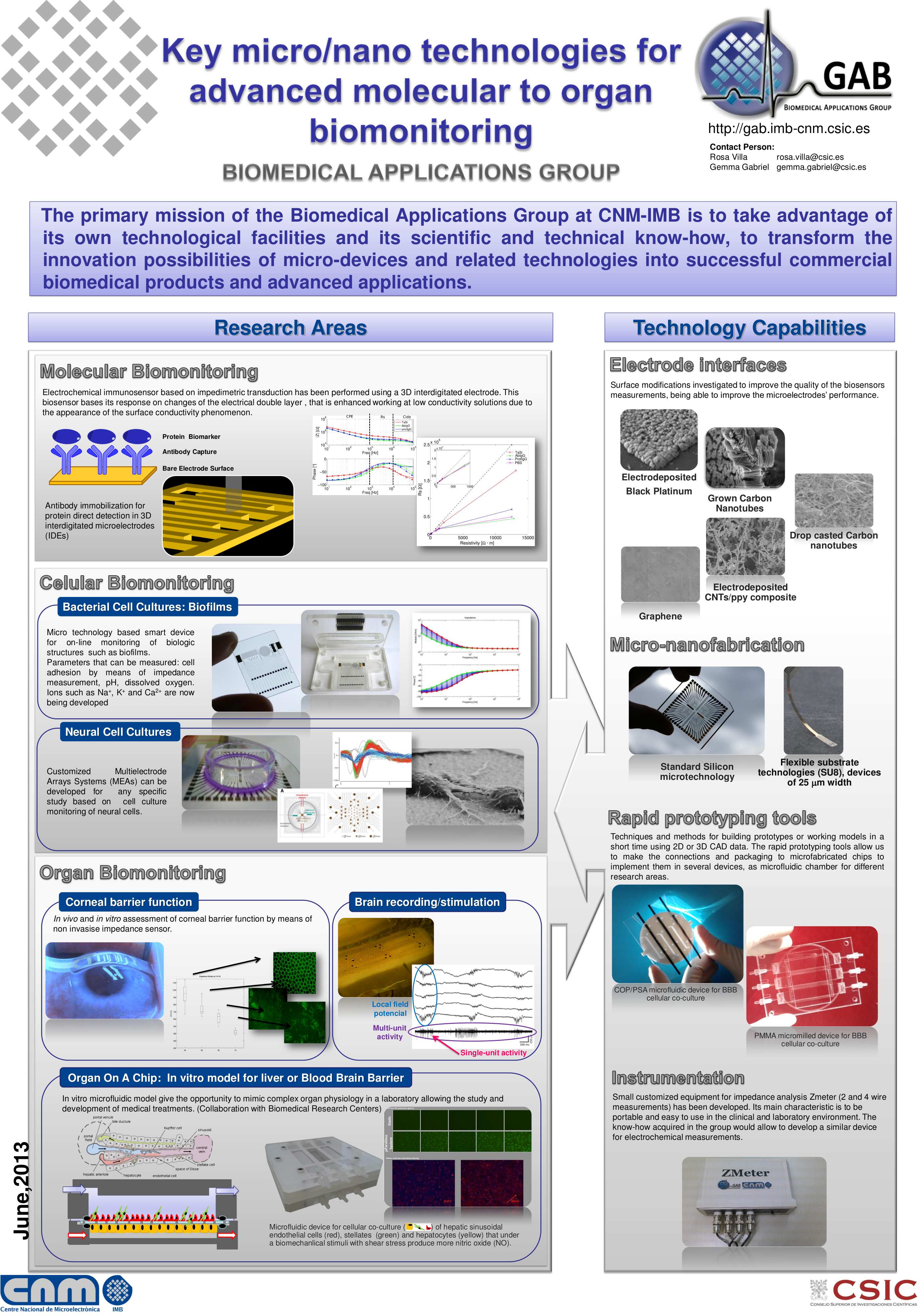
This thesis presents innovations aimed at enhancing in vitro human models and reducing reliance on animal testing. It focuses on the design of the devices and electrodes, material selection, and novel fabrication methods to monitor barrier function, model and measure oxygen levels, and detect neuronal electrophysiological activity. First, a microfluidic device with semitransparent electrodes was designed to monitor the integrity of barrier-forming tissues. The semitransparent electrodes allow to match the electrode area with the cell culture area for accurate measurements of barrier function while allowing optical access simultaneously. Second, a microfluidic device with adjustable oxygen scavenging capacity with integrated oxygen sensors was developed to better mimic the oxygen microenvironment of tissues for OoC applications. The sensor integration protocol was developed using inkjet printing technologies and can be adapted to various materials that are compatible with OoC technology. Third, the thesis introduces multi-sensing platforms that integrate multiple electrodes to enhance data acquisition from OoC systems, allowing for more detailed and robust information collection. This includes an OoC device that integrates both barrier function and oxygen level monitoring. Additionally, an origami-inspired platform was developed to sense the electrophysiological activity of neuronal 3D tissues while also monitoring barrier function. This thesis demonstrates the potential of integrating electrodes into advanced OoC devices to deepen our understanding of physiological processes and highlights the advantages of OoC over traditional models.